Abstract
Introduction
Complement-mediated intravascular hemolysis in patients with paroxysmal nocturnal hemoglobinuria (PNH) is effectively prevented by the terminal-complement inhibitor eculizumab, reducing symptoms and protecting against the clinical consequences of the disease, such as thrombosis and renal failure. Due to the continued activation of the proximal complement cascade upstream of C5, PNH red cells become opsonized with fragments of C3, leading to extravascular hemolysis and a continued transfusion requirement in a proportion of patients. Lactate dehydrogenase (LDH) is utilised as the main indicator of hemolysis in PNH but is particularly a measure of intravascular rather than extravascular hemolysis and therefore is not necessarily elevated in proportion to transfusion requirement due to C3-loading. We therefore conducted data analysis to determine whether other parameters were more indicative of extravascular hemolysis.
Methods
Data from patients with PNH treated in the Leeds centre of the UK PNH National Service was reviewed retrospectively. Criteria for inclusion in the analysis were treatment with eculizumab for ≥13 months, a mean platelet count >50 x 109/L and mean absolute neutrophil count >0.5 x 109/L (to exclude patients with significant bone marrow failure). Mean values for hemoglobin, absolute reticulocyte count, LDH (adjusted for upper limit of normal), total bilirubin and the proportion of PNH red cells loaded with C3 were collated covering a 48-month period. C3 loading of red cells was assessed by flow cytometry (Sutherland et al., Curr Protoc Cytom. 2015). Red cell transfusion requirements for the latest 12 months and latest eculizumab dosage were also collected.
Results
One hundred and forty-one patients were identified for inclusion by the above criteria; 70 males and 71 females. The median hemoglobin level for all patients was 109g/l. Seventy-two percent had a mean hemoglobin below 120g/l despite treatment with eculizumab and all patients had variable amounts of C3 on their red cells demonstrating that some degree of extravascular hemolysis is always seen. During the latest 12 months, 51 (36%) patients received at least one transfusion, with 23 (16%) requiring 3 or more transfusions. Thirty (21%) patients were receiving a higher dose of eculizumab (1200mg or more every 2 weeks) due to either sub-therapeutic eculizumab levels and/or measureable terminal complement levels, assessed by the CH50, at the standard dose of 900mg every two weeks.
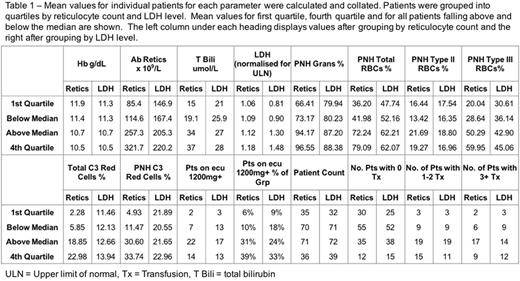
Patients were stratified by both reticulocyte count and LDH and the means for each parameter calculated as shown in Table 1. Bilirubin, C3 loading, transfusion requirement and requirement for an increased dose of eculizumab all correlated better when stratifying by reticulocytes rather than by LDH.
When patients were scored on the severity of C3 loading, bilirubin values and reticulocyte count, those with the worst (highest) joint scores were 3 times more likely to be transfused. 72/141 (51%) patients had all three of the following: reticulocyte count <230 x 109/l, bilirubin <30umol/l and less than 30% of their PNH red cells staining for C3. 55 of these 72 (76%) were not transfused in the last 12 months. In contrast 9 patients had reticulocytes >230, bilirubin ≥30 and C3-loading ≥30% and 7 of these 9 continued to require transfusions.
Conclusion
Reticulocyte count appears to be a better indicator of extravascular hemolysis than LDH, correlating more strongly with raised bilirubin, increased C3 loading of PNH red cells and increased transfusion requirement. Using these parameters patients who are more likely to continue to require transfusion support on eculizumab can be identified. Such an approach may be used to identify patients who are more likely to benefit from inhibition of the complement cascade that could reduce the level of C3-associated extravascular hemolysis.
Richards: Alexion Pharmaceuticals, Inc.: Honoraria. Munir: Alexion Pharmaceuticals, Inc.: Honoraria; Gilled: Honoraria; AbbVie: Honoraria; Janssen: Honoraria; Roche: Honoraria. Griffin: Alexion Pharmaceuticals, Inc.: Honoraria. Arnold: Alexion Pharmaceuticals, Inc.: Honoraria. Hill: Alexion Pharmaceuticals, Inc.: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. Hillmen: Roche: Consultancy, Honoraria, Research Funding; Celgene: Research Funding; Alexion Pharmaceuticals, Inc.: Consultancy, Honoraria; Pharmacyclics LLC, an AbbVie Company: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; Gilead: Consultancy, Honoraria, Research Funding; AbbVie: Consultancy, Honoraria, Research Funding; GSK: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal